Sodium Chloride State Of Matter At Room Temperature
What is the appearance of sodium chloride.
Sodium chloride state of matter at room temperature. Ringer s and sodium chloride irrigations may also be used for soaking surgical dressings instruments and laboratory specimens. What is the state of matter for magnesium at room temperature. Sodium is the sixth most common element on earth and makes up 2 6 of the earth s crust. What is the state of matter of nacl.
Sodium chloride can be melted to its liquid state at around 1200 k at room pressure. Nabr has a very low toxicity with an oral ld 50 estimated at 3 5 g kg for rats. Sodium chloride is the salt most responsible. Nacl is sodium chloride.
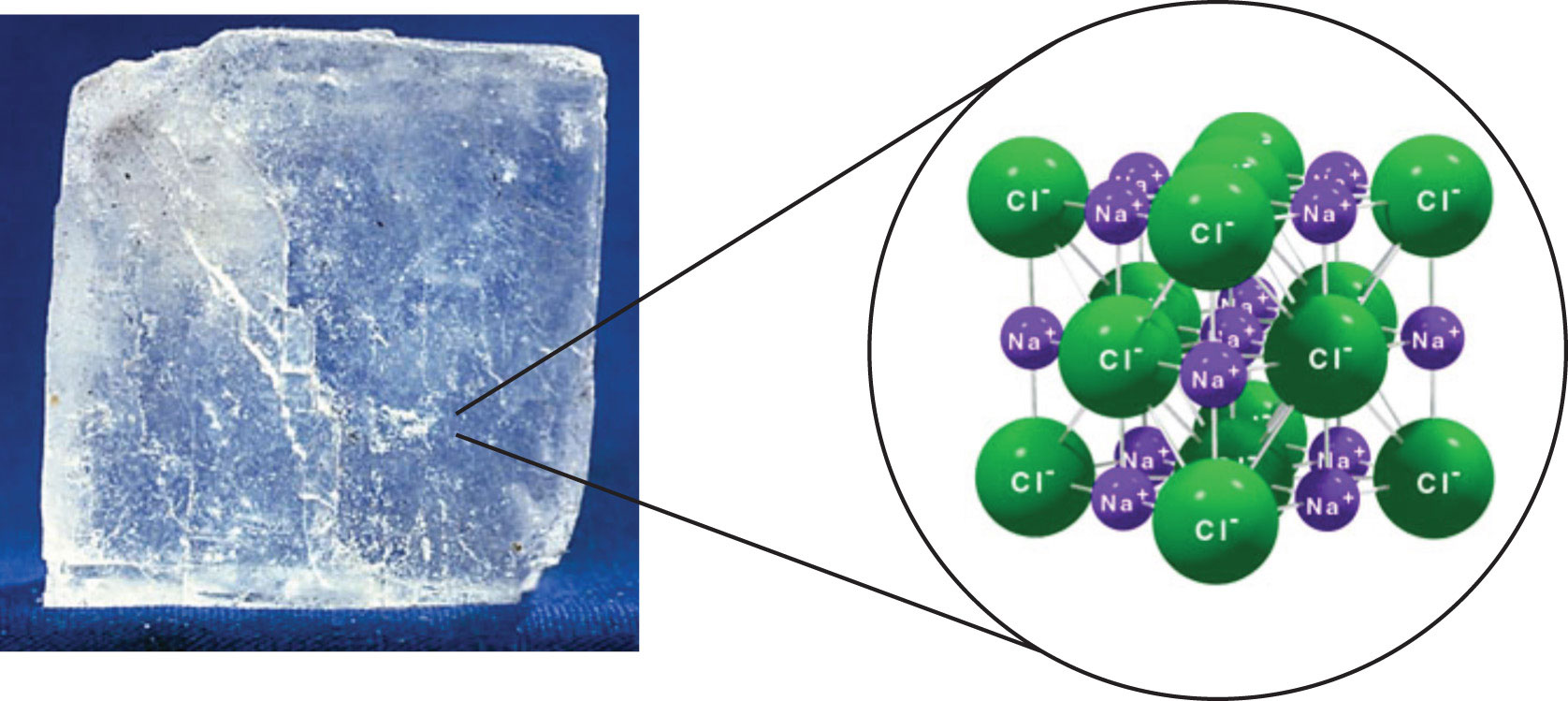
The melting boiling point of a substance determines what state. Learn more about the medical uses for salt. At the room temperature sodium chloride is a solid. Sodium is an important constituent of a number of silicate materials such as feldspars and micas.
This very soluble salt has been leached into the oceans over the lifetime of the planet but many salt beds or lakes are found where ancient seas have evaporated. What is the state of matter of lithium at 25 degrees celsius. However this is a single dose value. Table salt which is definitely a solid at room temperature is sodium chloride.
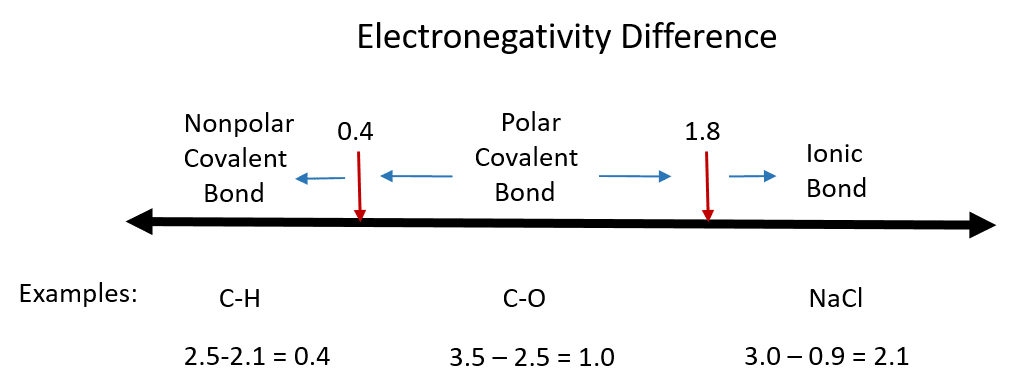
Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula nacl representing a 1 1 ratio of sodium and chloride ions. The most common compound is sodium chloride. Sodium bromide is used in conjunction with chlorine as a disinfectant for hot tubs and swimming pools. Sodium chloride also known as salt is used in medical treatments such as iv infusions and catheter flushes.
In 1807 sir humphry davy became the first to prepare sodium in its elemental form applying electrolysis to fused sodium hydroxide naoh. Because sodium is extremely reactive it never occurs in the free state in earth s crust. Sodium bromide is used to prepare dense fluids used in oil wells. A solid its melting point is 454 k 181 degrees c and its boiling point is 1620 k 1347 degrees c.
With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl.