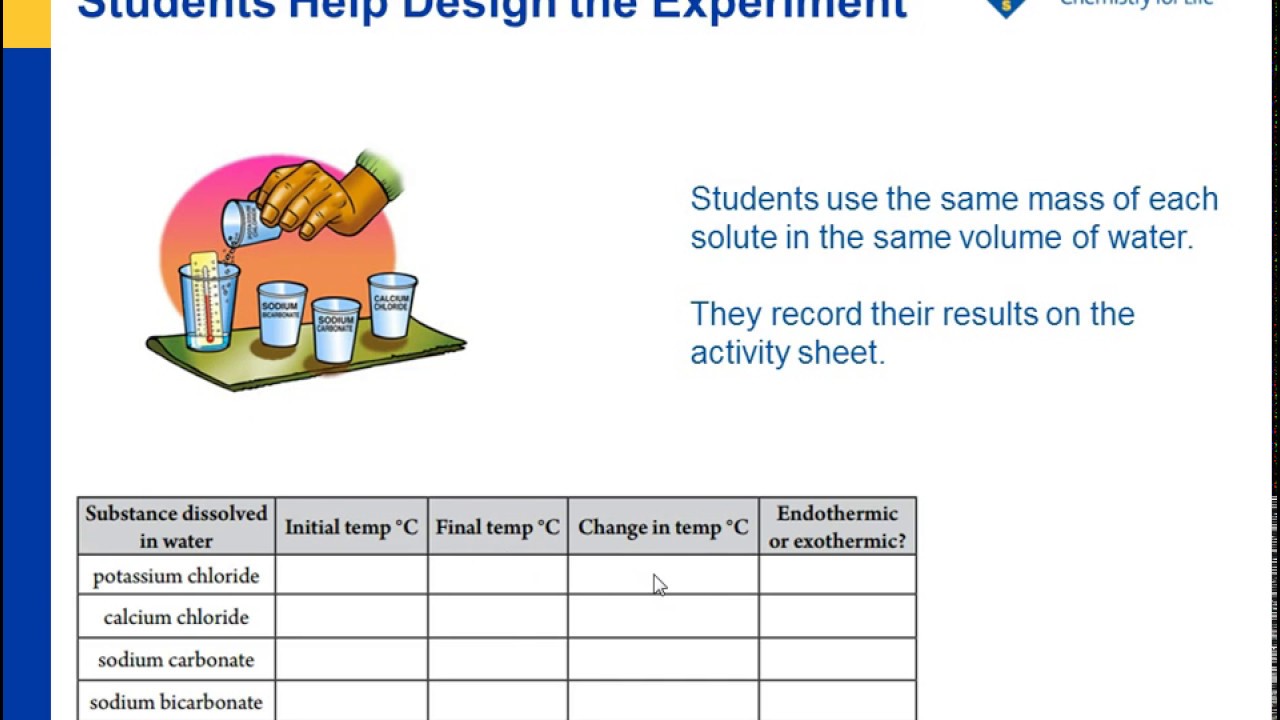
Solubility Of Epsom Salt In Water At Room Temperature

You should have noticed sugar had the highest solubility of all your tested compounds about 200 grams per 100 milliliters of water followed by epsom salts about 115 grams 100 milliliters table.
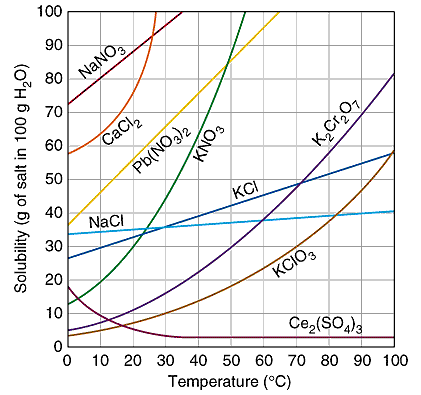
Solubility of epsom salt in water at room temperature. Some solutes exhibit solubility that is fairly independent of temperature. Solid solubility and temperature. Store at room temperature away from moisture and heat. The solubility of a given solute in a given solvent typically depends on temperature.
Epsom salt 10034 99 8 100 not established not established under the toxic substances control act tsca hydrates are considered as mixtures of their anhydrous salt and water. Hexahydrite mgso 4 6h 2 o as pure salt is only stable at a temperature range of approx. Ink in these markers is a color mixture. If the rh drops significantly lower than 50 at room temperature the chemically combined water is released and lower hydrate stages are formed.
This question has come up several times so here is a graph showing the saturation of mgso4 7 h2o magnesium sulfate heptahydrate epsom salt at various temperatures. Follow the directions on the product label about how much epsom salt to use per gallon of water. Try different water soluble markers. The substances are listed in alphabetical order.
Magnesium sulfate is usually encountered in the form of a hydrate mgso 4 nh 2 o for various values of n between 1 and 11. To the mixtu 258 22 680558 40 43212 17 75156 24 16284 30 57412 add the mass of the water to the mass of th 80 17 7 22 73 55 get the temperature data values from the sl 1 293 1 3 1 5 1 76 2 2 6 199 53596. Accordingly the cas numbers 7487 88 9 7732 18 5 are used for purposes of tsca. G salt 100 g water.
Many salts show a large increase in solubility with temperature. Black ink is composed. 48 c to 67 5 c. Since 1ml of water is 1 gram and the data is grams of epsom salt per 100ml of water the conversion of water to weight and the conversion of weights to percetage is very simple.
A few such as cerium iii sulfate become less soluble in water as temperature increases. Magnesium sulfate magnesium sulphate in british english is a chemical compound a salt with the formula mgso 4 consisting of magnesium cations mg 2 20 19 by mass and sulfate anions so 2 4 it is a white crystalline solid soluble in water but not in ethanol. The data shows the solubility of table salt and of epsom salts as temperature increases. To use epsom salt as an epsom salt soak dissolve in a large amount of water in a large bowl a bucket a foot tub or a bath tub.