Stainless Steel Electrodes Water Electrolysis

Some additional materials required.
Stainless steel electrodes water electrolysis. In modified ni electrodes cr 6 was detected within norm in the electrolyte. Chromium from electrode not oxidizing in electrolysis. Therefore when electricity is applied to the electrode the available current can be minimized 6 8. 0 40 copper 1 65 manganese 0 60 silicon deteriorates rusts and allows rust to stick regular cleaning once in 3 4 hours by a wire brush is required to deliver current during electrolysis.
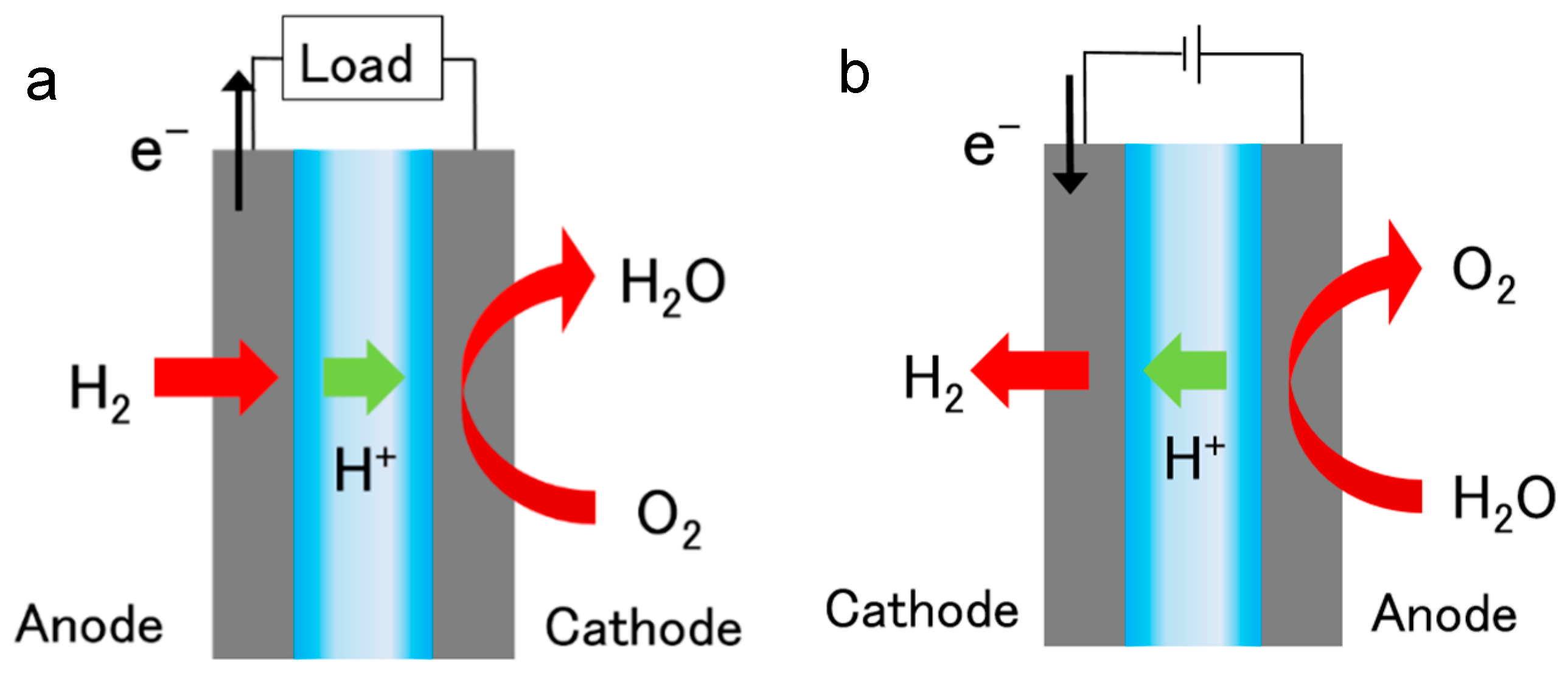
The cathode can be made of regular steel because electrolysis actually reverses black rust and will not form more corrosion in fact electrolysis is used to restore iron objects. The main problem with using it is the hazardous waste it produces. I wouldn t suggest copper including copper alloys or aluminum for either electrode because they corrode rapidly when used for electrolysis. Use this pair of stainless steel insulated electrodes to demonstrate electrolysis of water into hydrogen and oxygen.
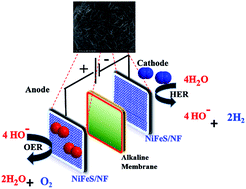
I know if i use stainless steel as the anode it does produce hexavalent chromium but what if it is a stainless cathode. Efficiency of electrolysis equipment can be improved by considering the effective surface of electrodes. Operations using such ss bearing materials proceed under substantial overpotentials above the thermodynamic requirement due to the sluggish kinetics of the anodic oxygen evolution reaction oer. One of the challenges to widespread use of water electrolysis is to reduce maintenance time.
Anode electrode for a water electrolyzer hand painted on stainless steel fiber paper. Overall even though mild steel is not as efficient as stainless steel it is a good choice for. Stainless steel contains chromium. Electrodes used in this research is a stainless steel 316 and naoh solution used as the electrolyte.
Dioxide materials offers one stop shopping experience for electrochemical equipment for co2 and water electrolysis and. Each anode catalyst is custom made and hand painted on stainless steel fiber paper. Stainless steel ss has been widely incorporated into electrodes or used as a conductive scaffold in industrial water electrolysis. 4 mild steel 2 1 carbon.
Cr 6 is very dangerous metal ion generates different types of cancer in people. Cr 6 was detected out of norm in electrolyte using stainless steel electrodes. Stainless steel is indeed consumed when used in the electrolysis process although slowly. I want to do rust removal by electrolysis sodium carbonate water solution can i use a stainless steel basket as a cathode or will it produce hexavalent chromium.
Place an order dioxide materials navigation.